|
|
АкушерствоАнатомияАнестезиологияВакцинопрофилактикаВалеологияВетеринарияГигиенаЗаболеванияИммунологияКардиологияНеврологияНефрологияОнкологияОториноларингологияОфтальмологияПаразитологияПедиатрияПервая помощьПсихиатрияПульмонологияРеанимацияРевматологияСтоматологияТерапияТоксикологияТравматологияУрологияФармакологияФармацевтикаФизиотерапияФтизиатрияХирургияЭндокринологияЭпидемиология |
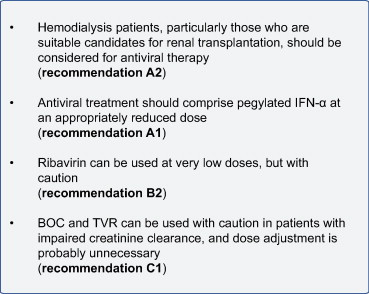
ПАЦИЕНТОВ, НАХОДЯЩИХСЯ НА ГЕМОДИАЛИЗЕИнфекция ВГС превалирует в гемодиализе населения и связан с повышенным риском для всех-причины и печеночно-связанной смертности. Сердечно-сосудистые заболевания по-прежнему, однако, основной причиной смерти у диализных пациентов независимо от статуса ВГС. Как и во всех настройках, кандидатуру диализных пациентов для противовирусной терапии требует специального рассмотрения сопутствующих заболеваний, поскольку болезнь печени может оказать незначительное влияние на прогнозируемые показатели заболеваемости и смертности этого пациента. ВГС-ассоциированного поражения печени может быть ускорен путем иммуносупрессии, и ИФН-α может привести к развитию почечной отторжение трансплантата. По этим причинам, противовирусная терапия должна быть рассмотрена для всех пациентов, находящихся на гемодиализе, которые будут кандидатами для трансплантации почки. Отражая опасения по поводу применения рибавирина в эту настройку, большинство опубликованных данных описывают использование ИФН-α монотерапии, в основном в небольших исследованиях с использованием обычных ИФН-α [154]. Пегилированный ИФН-α могут быть использованы и могут быть связаны с улучшением УВО [[155], [156]]. Пегилированный ИФН-α аккумулируется у пациентов с прогрессирующей почечной дисфункции, поэтому снижение дозы не требуется. Рекомендуемая доза Пег ИФН α 2а в этот параметр составляет 135 мкг/неделю. Обработка комбинации с Пегифн/РИБАВИРИН можно считать опытными врачами, и может повысить уровень УВО [157]. Индивидуализированное дозирование рибавирина по 200 мг/сут или 200 мг/через день или 200 мг трижды в неделю после гемодиализа, кроветворной и существенная поддержка необходима. Фармакокинетические исследования у пациентов с терминальной стадией почечной недостаточности выявляют существенного влияния почечной дисфункции на медикаментозное воздействие, предполагая, что оба ТВР и boc может быть использовано для лечения HCV-инфекции в этой обстановке [[158], [159]]. Нет опубликованных данных для описания эффективности и безопасности ПИ-включительно противовирусное лечение почечной недостаточности у пациентов с ВГС, так что клинические исследования в этой группе населения являются существенными. Недавно представила исследование, в котором участвовали 36 лечение-наивно Генотип 1 гемодиализных больных показал, что ТВР-содержащая тройная терапия имела более высокую эффективность, чем Пегифн/РИБАВИРИН-двойной терапии, но тройной терапии была связана с более анемия [160]. РЕКОМЕНДАЦИИ
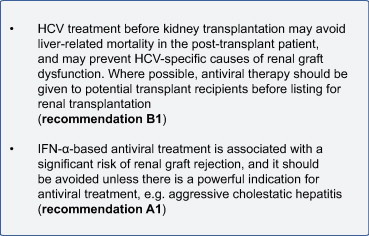
НЕ ПЕЧЕНИ РЕЦИПИЕНТОВ ТРАНСПЛАНТАТОВ СОЛИДНЫХ ОРГАНОВ HCV-инфекции у реципиентов почечного трансплантата может ассоциироваться с повышением темпов прогрессирования фиброза печени. Большинство исследований по пересадке почки когорты показывают, что ВГС-позитивности связана с нарушением почечного трансплантата и выживаемость пациентов. Нарушениями зрения выживаемости трансплантата частично отражает увеличение смертности больных. Кроме того, ВГС специфических причин, связанных, таких как гломерулонефрит и повышенным риском диабета прививка повлияет на исход. ВГС-позитивности связана с повышенным от всех причин и печеночно-связанной смертности, хотя сердечно-сосудистые заболевания остаются основной причиной смерти пациента [161]. Как цирроз печени является важным предиктором плохого после трансплантации выживаемость после трансплантации почки, желательно сделать оценку стадии фиброза печени во всех ВГС-позитивных пересадки почки кандидатов [162]. Для пациентов с диагностированным циррозом печени, которым не удается (или непригодны к) гепатита противовирусное лечение, изолированной трансплантации почки может быть противопоказана и следует рассмотреть вопрос о комбинированной трансплантации печени и почек [163]. Лечение хронического гепатита С Пегифн/РИБАВИРИН у реципиентов почечного трансплантата связана с риском острого или хронического клеточного отторжения от 30% и более, в результате чего потери трансплантата и выживаемость пациентов снижена. Поэтому, Пегифн/РИБАВИРИН терапии имеет дополнительный риск в таких больных, и решение дать противовирусной терапии должны учитывать эти риски. Там, где это возможно, пациенты с показанием к трансплантации почки надо лечить от гепатита с. перед трансплантацией [164]. Данные об HCV-инфекции после трансплантации сердца немногочисленны и противоречивы исследования, показывающие без изменений или снизился уровень выживаемости у пациентов, инфицированных ВГС. Никаких исследований о рисках и преимуществах противовирусной терапии доступны для этих пациентов, а риск отторжения трансплантата на ИФН-α лечения остается неясной. В этом контексте лечения хронической HCV-инфекции в сердце реципиентов не может быть рекомендована и показания должны оцениваться на индивидуальной основе, если ВГС-инфекция представляет угрозу для жизни. Международные руководящие принципы список хронической HCV-инфекции как противопоказание к трансплантации легких [165]. Лечение легочного трансплантата до пересадки кандидатов был рекомендован некоторыми авторами, но существует ограниченный опыт с таким подходом. Нет данных о влиянии HCV-инфекции и ее лечение после трансплантации поджелудочной железы или тонкой кишки. РЕКОМЕНДАЦИИ
REFERENCES [1]EASL Clinical Practice Guidelines. Management of hepatitis C virus infection. J Hepatol. 2011; 55: 245264 [2]Lavanchy, D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011; 17: 107115 [3]Hepatitis, C. Wkly Epidemiol Rec. 1997; 72: 6569 [4]Cornberg, M., Razavi, H.A., Alberti, A., Bernasconi, E., Buti, M., Cooper, C. et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011; 31: 3060 [5]Blachier M, Leleu H, Peck-Radosavljevic M, Valla D-C, Roudot-Thoraval F. The burden of liver disease in Europe, a review of available epidemiological data. Geneva: European Association for the Study of the, Liver; 2013. www.easl.eu. [6]Rantala M, van de Laar M. Surveillance and epidemiology of hepatitis B and C in Europe a review. Eur Surveill 2008;13(21): <http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18880>. [7]Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, et al. Expanded classification of hepatitis C Virus into 7 genotypes and 67 Subtypes: updated criteria and assignment web resource. Hepatology 2013. http://dx.doi.org/10.1002/hep.26744 [Epub ahead of print, PubMed PMID: 24115039.]. [8]Antaki, N., Craxi, A., Kamal, S., Moucari, R., Van der Merwe, S., Haffar, S. et al. The neglected hepatitis C virus genotypes 4, 5, and 6: an international consensus report. Liver Int. 2010; 30: 342355 [9]Murphy, D., Chamberland, J., Dandavino, R., and Sablon, E. A new genotype of hepatitis C virus originating from central Africa. Hepatology. 2007; 46: 623A [10]Martin, N.K., Vickerman, P., Foster, G.R., Hutchinson, S.J., Goldberg, D.J., and Hickman, M. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J Hepatol. 2011; 54: 11371144 [11]van de Laar, T.J., Matthews, G.V., Prins, M., and Danta, M. Acute hepatitis C in HIV-infected men who have sex with men: an emerging sexually transmitted infection. AIDS. 2010; 24: 17991812 [12]Esteban, J.I., Sauleda, S., and Quer, J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008; 48: 148162 [13]Thein, H.H., Yi, Q., Dore, G.J., and Krahn, M.D. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008; 48: 418431 [14]John-Baptiste, A., Krahn, M., Heathcote, J., Laporte, A., and Tomlinson, G. The natural history of hepatitis C infection acquired through injection drug use: meta-analysis and meta-regression. J Hepatol. 2010; 53: 245251 [15]Yang, J.D. and Roberts, L.R. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010; 7: 448458 [16]Hepatitis B and C in the EU neighbourhood: prevalence, burden of disease and screening policies. Stockholm: European Centre for Disease Prevention and Control; 2010. [17]McDonald, S.A., Hutchinson, S.J., Bird, S.M., Mills, P.R., Dillon, J., Bloor, M. et al. A population-based record linkage study of mortality in hepatitis C-diagnosed persons with or without HIV coinfection in Scotland. Stat Methods Med Res. 2009; 18: 271283 [18]Grebely, J. and Dore, G.J. What is killing people with hepatitis C virus infection?. Semin Liver Dis. 2011; 31: 331339 [19]Seeff, L.B. The history of the natural history of hepatitis C (19682009). Liver Int. 2009; 29: 8999 [20]Mallat, A., Hezode, C., and Lotersztajn, S. Environmental factors as disease accelerators during chronic hepatitis C. J Hepatol. 2008; 48: 657665 [21]Brunet, L., Moodie, E.E., Rollet, K., Cooper, C., Walmsley, S., Potter, M. et al. Marijuana smoking does not accelerate progression of liver disease in HIV-hepatitis C coinfection: a longitudinal cohort analysis. Clin Infect Dis. 2013; 57: 663670 [22]Costentin, C.E., Roudot-Thoraval, F., Zafrani, E.S., Medkour, F., Pawlotsky, J.M., Mallat, A. et al. Association of caffeine intake and histological features of chronic hepatitis C. J Hepatol. 2011; 54: 11231129 [23]Modi, A.A., Feld, J.J., Park, Y., Kleiner, D.E., Everhart, J.E., Liang, T.J. et al. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010; 51: 201209 [24]Ohfuji, S., Fukushima, W., Tanaka, T., Habu, D., Tamori, A., Sakaguchi, H. et al. Coffee consumption and reduced risk of hepatocellular carcinoma among patients with chronic type C liver disease: a case-control study. Hepatol Res. 2006; 36: 201208 [25]Manns, M.P. and von Hahn, T. Novel therapies for hepatitis C one pill fits all?. Nat Rev Drug Discov. 2013; 12: 595610 [26]Andrews, J., Guyatt, G., Oxman, A.D., Alderson, P., Dahm, P., Falck-Ytter, Y. et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013; 66: 719725 [27]Chevaliez, S. and Pawlotsky, J.M. Diagnosis and management of chronic viral hepatitis: antigens, antibodies and viral genomes. Best Pract Res Clin Gastroenterol. 2008; 22: 10311048 [28]Kamili, S., Drobeniuc, J., Araujo, A.C., and Hayden, T.M. Laboratory diagnostics for hepatitis C virus infection. Clin Infect Dis. 2012; 55: S43S48 [29]Swain, M.G., Lai, M.Y., Shiffman, M.L., Cooksley, W.G., Zeuzem, S., Dieterich, D.T. et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010; 139: 15931601 [30]Martinot-Peignoux, M., Stern, C., Maylin, S., Ripault, M.P., Boyer, N., Leclere, L. et al. Twelve weeks posttreatment follow-up is as relevant as 24 weeks to determine the sustained virologic response in patients with hepatitis C virus receiving pegylated interferon and ribavirin. Hepatology. 2010; 51: 11221126 [31]Castera, L., Vergniol, J., Foucher, J., Le Bail, B., Chanteloup, E., Haaser, M. et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005; 128: 343350 [32]Castera, L., Sebastiani, G., Le Bail, B., de Ledinghen, V., Couzigou, P., and Alberti, A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010; 52: 191198 [33]Chevaliez, S., Bouvier-Alias, M., Brillet, R., and Pawlotsky, J.M. Hepatitis C virus (HCV) genotype 1 subtype identification in new HCV drug development and future clinical practice. PLoS One. 2009; 4: e8209 [34]Thompson, A.J., Muir, A.J., Sulkowski, M.S., Ge, D., Fellay, J., Shianna, K.V. et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010; 139: e118 [35]Ge, D., Fellay, J., Thompson, A.J., Simon, J.S., Shianna, K.V., Urban, T.J. et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009; 461: 399401 [36]Hezode, C., Fontaine, H., Dorival, C., Larrey, D., Zoulim, F., Canva, V. et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) NCT01514890. J Hepatol. 2013; 59: 434441 [37]Poordad, F., McCone, J. Jr., Bacon, B.R., Bruno, S., Manns, M.P., Sulkowski, M.S. et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011; 364: 11951206 [38]Jacobson, I.M., McHutchison, J.G., Dusheiko, G., Di Bisceglie, A.M., Reddy, K.R., Bzowej, N.H. et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011; 364: 24052416 [39]Sherman, K.E., Flamm, S.L., Afdhal, N.H., Nelson, D.R., Sulkowski, M.S., Everson, G.T. et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011; 365: 10141024 [40]Fried, M.W., Shiffman, M.L., Reddy, K.R., Smith, C., Marinos, G., Goncales, F.L. Jr. et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002; 347: 975982 [41]Hadziyannis, S.J., Sette, H. Jr., Morgan, T.R., Balan, V., Diago, M., Marcellin, P. et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004; 140: 346355 [42]Manns, M.P., Wedemeyer, H., and Cornberg, M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006; 55: 13501359 [43]McHutchison, J.G., Lawitz, E.J., Shiffman, M.L., Muir, A.J., Galler, G.W., McCone, J. et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009; 361: 580593 [44]Poordad, F., Bronowicki, J.P., Gordon, S.C., Zeuzem, S., Jacobson, I.M., Sulkowski, M.S. et al. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012; 143: e601e605 [45]Buti M, Agarwal K, Horsmans YJ, Sievert W, Janczewska E, Zeuzem S, et al. OPTIMIZE trial: non-inferiority of twice-daily telaprevir vs. administration every 8 h in treatment-naive, genotype 1 HCV infected patients. In: 63rd annual meeting of the American Association for the Study of Liver Diseases, Boston, MA, November 913; 2012 [abstract LB8]. [46]Marcellin, P., Forns, X., Goeser, T., Ferenci, P., Nevens, F., Carosi, G. et al. Telaprevir is effective given every 8 or 12 h with ribavirin and peginterferon alfa-2a or -2b to patients with chronic hepatitis C. Gastroenterology. 2011; 140: 459468 ([quiz e414]) [47]Manns, M., Zeuzem, S., Sood, A., Lurie, Y., Cornberg, M., Klinker, H. et al. Reduced dose and duration of peginterferon alfa-2b and weight-based ribavirin in patients with genotype 2 and 3 chronic hepatitis C. J Hepatol. 2011; 55: 554563 [48]Marcellin, P., Cheinquer, H., Curescu, M., Dusheiko, G.M., Ferenci, P., Horban, A. et al. High sustained virologic response rates in rapid virologic response patients in the large real-world PROPHESYS cohort confirm results from randomized clinical trials. Hepatology. 2012; 56: 20392050 [49]De Nicola, S., Aghemo, A., Rumi, M.G., Galmozzi, E., Valenti, L., Soffredini, R. et al. Interleukin 28B polymorphism predicts pegylated interferon plus ribavirin treatment outcome in chronic hepatitis C genotype 4. Hepatology. 2012; 55: 336342 [50]Vermehren, J., Kau, A., Gartner, B.C., Gobel, R., Zeuzem, S., and Sarrazin, C. Differences between two real-time PCR-based hepatitis C virus (HCV) assays (RealTime HCV and Cobas AmpliPrep/Cobas TaqMan) and one signal amplification assay (Versant HCV RNA 3.0) for RNA detection and quantification. J Clin Microbiol. 2008; 46: 38803891 [51]Chevaliez, S., Bouvier-Alias, M., Brillet, R., and Pawlotsky, J.M. Overestimation and underestimation of hepatitis C virus RNA levels in a widely used real-time polymerase chain reaction-based method. Hepatology. 2007; 46: 2231 [52]Sarrazin, C., Shiffman, M.L., Hadziyannis, S.J., Lin, A., Colucci, G., Ishida, H. et al. Definition of rapid virologic response with a highly sensitive real-time PCR-based HCV RNA assay in peginterferon alfa-2a plus ribavirin response-guided therapy. J Hepatol. 2010; 52: 832838 [53]Manns, M.P., McHutchison, J.G., Gordon, S.C., Rustgi, V.K., Shiffman, M., Reindollar, R. et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001; 358: 958965 [54]Diago, M., Shiffman, M.L., Bronowicki, J.P., Zeuzem, S., Rodriguez-Torres, M., Pappas, S.C. et al. Identifying hepatitis C virus genotype 2/3 patients who can receive a 16-week abbreviated course of peginterferon alfa-2a (40KD) plus ribavirin. Hepatology. 2010; 51: 18971903 [55]Shiffman, M.L., Suter, F., Bacon, B.R., Nelson, D., Harley, H., Sola, R. et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007; 357: 124134 [56]Jensen, D.M., Morgan, T.R., Marcellin, P., Pockros, P.J., Reddy, K.R., Hadziyannis, S.J. et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology. 2006; 43: 954960 [57]Ferenci, P., Laferl, H., Scherzer, T.M., Gschwantler, M., Maieron, A., Brunner, H. et al. Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology. 2008; 135: 451458 [58]Berg, T., Sarrazin, C., Herrmann, E., Hinrichsen, H., Gerlach, T., Zachoval, R. et al. Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology. 2003; 37: 600609 [59]Zeuzem, S., Buti, M., Ferenci, P., Sperl, J., Horsmans, Y., Cianciara, J. et al. Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol. 2006; 44: 97103 [60]Davis, G.L., Wong, J.B., McHutchison, J.G., Manns, M.P., Harvey, J., and Albrecht, J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003; 38: 645652 [61]Mangia, A., Minerva, N., Bacca, D., Cozzolongo, R., Ricci, G.L., Carretta, V. et al. Individualized treatment duration for hepatitis C genotype 1 patients: a randomized controlled trial. Hepatology. 2008; 47: 4350 [62]Moreno, C., Deltenre, P., Pawlotsky, J.M., Henrion, J., Adler, M., and Mathurin, P. Shortened treatment duration in treatment-naive genotype 1 HCV patients with rapid virological response: a meta-analysis. J Hepatol. 2010; 52: 2531 [63]Berg, T., von Wagner, M., Nasser, S., Sarrazin, C., Heintges, T., Gerlach, T. et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 vs. 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006; 130: 10861097 [64]Pearlman, B.L., Ehleben, C., and Saifee, S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis C genotype 1-infected slow responders. Hepatology. 2007; 46: 16881694 [65]Sanchez-Tapias, J.M., Diago, M., Escartin, P., Enriquez, J., Romero-Gomez, M., Barcena, R. et al. Peginterferon-alfa2a plus ribavirin for 48 vs. 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology. 2006; 131: 451460 [66]Ferenci, P., Laferl, H., Scherzer, T.M., Maieron, A., Hofer, H., Stauber, R. et al. Peginterferon alfa-2a/ribavirin for 48 or 72 weeks in hepatitis C genotypes 1 and 4 patients with slow virologic response. Gastroenterology. 2010; 138: 503512 ([512 e501]) [67]Buti, M., Lurie, Y., Zakharova, N.G., Blokhina, N.P., Horban, A., Teuber, G. et al. Randomized trial of peginterferon alfa-2b and ribavirin for 48 or 72 weeks in patients with hepatitis C virus genotype 1 and slow virologic response. Hepatology. 2010; 52: 12011207 [68]Farnik, H., Lange, C.M., Sarrazin, C., Kronenberger, B., Zeuzem, S., and Herrmann, E. Meta-analysis shows extended therapy improves response of patients with chronic hepatitis C virus genotype 1 infection. Clin Gastroenterol Hepatol. 2010; 8: 884890 [69]Dalgard, O., Bjoro, K., Ring-Larsen, H., Bjornsson, E., Holberg-Petersen, M., Skovlund, E. et al. Pegylated interferon alfa and ribavirin for 14 vs. 24 weeks in patients with hepatitis C virus genotype 2 or 3 and rapid virological response. Hepatology. 2008; 47: 3542 [70]Mangia, A., Santoro, R., Minerva, N., Ricci, G.L., Carretta, V., Persico, M. et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005; 352: 26092617 [71]von Wagner, M., Huber, M., Berg, T., Hinrichsen, H., Rasenack, J., Heintges, T. et al. Peginterferon-alpha-2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology. 2005; 129: 522527 [72]Yu, M.L., Dai, C.Y., Huang, J.F., Hou, N.J., Lee, L.P., Hsieh, M.Y. et al. A randomised study of peginterferon and ribavirin for 16 vs. 24 weeks in patients with genotype 2 chronic hepatitis C. Gut. 2007; 56: 553559 [73]Kau, A., Vermehren, J., and Sarrazin, C. Treatment predictors of a sustained virologic response in hepatitis B and C. J Hepatol. 2008; 49: 634651 [74]Romero-Gomez, M., Fernandez-Rodriguez, C.M., Andrade, R.J., Diago, M., Alonso, S., Planas, R. et al. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008; 48: 721727 [75]Zeuzem, S., Hultcrantz, R., Bourliere, M., Goeser, T., Marcellin, P., Sanchez-Tapias, J. et al. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004; 40: 993999 [76]Berg, T., Shiffman, M.L., Zeuzem, S., Berg, C.P., de Figueiredo-Mendes, C., Dore, G.J. et al. 48 Weeks of peginterferon alfa-2a/ribavirin improves SVR24 and decreases relapse across HCV genotype 2/3 patient subgroups not achieving a rapid virological response: N-CORE study. J Hepatol. 2013; 58: S323 [77]Fried, M.W. Side effects of therapy of hepatitis C and their management. Hepatology. 2002; 36: S237S244 [78]Soza, A., Everhart, J.E., Ghany, M.G., Doo, E., Heller, T., Promrat, K. et al. Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology. 2002; 36: 12731279 [79]Shiffman, M.L., Salvatore, J., Hubbard, S., Price, A., Sterling, R.K., Stravitz, R.T. et al. Treatment of chronic hepatitis C virus genotype 1 with peginterferon, ribavirin, and epoetin alpha. Hepatology. 2007; 46: 371379 [80]Afdhal, N.H., Dieterich, D.T., Pockros, P.J., Schiff, E.R., Shiffman, M.L., Sulkowski, M.S. et al. Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study. Gastroenterology. 2004; 126: 13021311 [81]Pockros, P.J., Shiffman, M.L., Schiff, E.R., Sulkowski, M.S., Younossi, Z., Dieterich, D.T. et al. Epoetin alfa improves quality of life in anemic HCV-infected patients receiving combination therapy. Hepatology. 2004; 40: 14501458 [82]Sulkowski, M.S., Poordad, F., Manns, M.P., Bronowicki, J.P., Rajender Reddy, K., Harrison, S.A. et al. Anemia during treatment with peginterferon Alfa-2b/ribavirin and boceprevir: analysis from the serine protease inhibitor therapy 2 (SPRINT-2) trial. Hepatology. 2013; 57: 974984 [83]Weiss, J.J., Brau, N., Stivala, A., Swan, T., and Fishbein, D. Review article: adherence to medication for chronic hepatitis C building on the model of human immunodeficiency virus antiretroviral adherence research. Aliment Pharmacol Ther. 2009; 30: 1427 [84]Grebely, J., Matthews, G.V., Hellard, M., Shaw, D., van Beek, I., Petoumenos, K. et al. Adherence to treatment for recently acquired hepatitis C virus (HCV) infection among injecting drug users. J Hepatol. 2011; 55: 7685 [85]Marcellin, P., Chousterman, M., Fontanges, T., Ouzan, D., Rotily, M., Varastet, M. et al. Adherence to treatment and quality of life during hepatitis C therapy: a prospective, real-life, observational study. Liver Int. 2011; 31: 516524 [86]Smith, S.R., Wahed, A.S., Kelley, S.S., Conjeevaram, H.S., Robuck, P.R., and Fried, M.W. Assessing the validity of self-reported medication adherence in hepatitis C treatment. Ann Pharmacother. 2007; 41: 11161123 [87]Evon, D.M., Simpson, K., Kixmiller, S., Galanko, J., Dougherty, K., Golin, C. et al. A randomized controlled trial of an integrated care intervention to increase eligibility for chronic hepatitis C treatment. Am J Gastroenterol. 2011; 106: 17771786 [88]Grebely, J., Knight, E., Genoway, K.A., Viljoen, M., Khara, M., Elliott, D. et al. Optimizing assessment and treatment for hepatitis C virus infection in illicit drug users: a novel model incorporating multidisciplinary care and peer support. Eur J Gastroenterol Hepatol. 2010; 22: 270277 [89]Arora, S., Thornton, K., Murata, G., Deming, P., Kalishman, S., Dion, D. et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011; 364: 21992207 [90]Larrey, D., Salse, A., Ribard, D., Boutet, O., Hyrailles-Blanc, V., Niang, B. et al. Education by a nurse increases response of patients with chronic hepatitis C to therapy with peginterferon-alpha2a and ribavirin. Clin Gastroenterol Hepatol. 2011; 9: 781785 [91]Schmidt, C., Schulte, B., Gansefort, D., Goelz, J., Gerken, G., Scherbaum, N. et al. Optimizing HCV therapy: the impact of psychoeducation on retention and SVR in opiate substituted patients. Hepatology. 2011; 54: 821A822A [92]Lindenburg, C.E., Lambers, F.A., Urbanus, A.T., Schinkel, J., Jansen, P.L., Krol, A. et al. Hepatitis C testing and treatment among active drug users in Amsterdam: results from the DUTCH-C project. Eur J Gastroenterol Hepatol. 2011; 23: 2331 [93]Waizmann, M. and Ackermann, G. High rates of sustained virological response in hepatitis C virus-infected injection drug users receiving directly observed therapy with peginterferon alpha-2a (40KD) (PEGASYS) and once-daily ribavirin. J Subst Abuse Treat. 2010; 38: 338345 [94]Grebely, J., Raffa, J.D., Meagher, C., Duncan, F., Genoway, K.A., Khara, M. et al. Directly observed therapy for the treatment of hepatitis C virus infection in current and former injection drug users. J Gastroenterol Hepatol. 2007; 22: 15191525 [95]Bonkovsky, H.L., Tice, A.D., Yapp, R.G., Bodenheimer, H.C. Jr., Monto, A., Rossi, S.J. et al. Efficacy and safety of peginterferon alfa-2a/ribavirin in methadone maintenance patients: randomized comparison of direct observed therapy and self-administration. Am J Gastroenterol. 2008; 103: 27572765 [96]Sylvestre, D.L. and Zweben, J.E. Integrating HCV services for drug users: a model to improve engagement and outcomes. Int J Drug Policy. 2007; 18: 406410 [97]Norman, J., Walsh, N.M., Mugavin, J., Stoove, M.A., Kelsall, J., Austin, K. et al. The acceptability and feasibility of peer worker support role in community based HCV treatment for injecting drug users. Harm Reduct J. 2008; 5: 8 [98]Rodis, J.L. and Kibbe, P. Evaluation of medication adherence and quality of life in patients with hepatitis C virus receiving combination therapy. Gastroenterol Nurs. 2010; 33: 368373 [99]Alavian, S.M. and Aalaei-Andabili, S.H. Education by a nurse increases the adherence to therapy in chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2012; 10: 203 ([author reply 203]) [100]Weiss, J.J., Alcorn, M.C., Rabkin, J.G., and Dieterich, D.T. The critical role of medication adherence in the success of boceprevir and telaprevir in clinical practice. J Hepatol. 2012; 56: 503504 [101]Bressler, B.L., Guindi, M., Tomlinson, G., and Heathcote, J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003; 38: 639644 [102]Anand, B.S., Currie, S., Dieperink, E., Bini, E.J., Shen, H., Ho, S.B. et al. Alcohol use and treatment of hepatitis C virus: results of a national multicenter study. Gastroenterology. 2006; 130: 16071616 [103]Le Lan, C., Guillygomarch, A., Danielou, H., Le Dreau, G., Laine, F., Vedeilhie, C. et al. A multi-disciplinary approach to treating hepatitis C with interferon and ribavirin in alcohol-dependent patients with ongoing abuse. J Hepatol. 2012; 56: 334340 [104]Bruggmann, P., Dampz, M., Gerlach, T., Kravecz, L., and Falcato, L. Treatment outcome in relation to alcohol consumption during hepatitis C therapy: an analysis of the Swiss Hepatitis C Cohort Study. Drug Alcohol Depend. 2010; 110: 167171 [105]Siu, L., Foont, J., and Wands, J.R. Hepatitis C virus and alcohol. Semin Liver Dis. 2009; 29: 188199 [106]Serfaty, L., Forns, X., Goeser, T., Ferenci, P., Nevens, F., Carosi, G. et al. Insulin resistance and response to telaprevir plus peginterferon alpha and ribavirin in treatment-naive patients infected with HCV genotype 1. Gut. 2012; 61: 14731480 [107]Harrison, S.A., Hamzeh, F.M., Han, J., Pandya, P.K., Sheikh, M.Y., and Vierling, J.M. Chronic hepatitis C genotype 1 patients with insulin resistance treated with pioglitazone and peginterferon alpha-2a plus ribavirin. Hepatology. 2012; 56: 464473 [108]Thevenot, T., Cadranel, J.F., Di Martino, V., Pariente, A., Causse, X., Renou, C. et al. A national French survey on the use of growth factors as adjuvant treatment of chronic hepatitis C. Hepatology. 2007; 45: 377383 [109]Stickel, F., Helbling, B., Heim, M., Geier, A., Hirschi, C., Terziroli, B. et al. Critical review of the use of erythropoietin in the treatment of anaemia during therapy for chronic hepatitis C. J Viral Hepat. 2012; 19: 7787 [110]Alavian, S.M., Tabatabaei, S.V., and Behnava, B. Impact of erythropoietin on sustained virological response to peginterferon and ribavirin therapy for HCV infection: a systematic review and meta-analysis. J Viral Hepat. 2012; 19: 8893 [111]Poordad, F., Lawitz, E.J., Reddy, K.R., Afdhal, N.H., Hzode, C., Zeuzem, S. et al. A randomized trial comparing ribavirin dose reduction vs. erythropoietin for anemia management in previously untreated patients with chronic hepatitis C receiving boceprevir plus peginterferon/ribavirin. J Hepatol. 2012; 56: S559 [112]Tandon, P., Doucette, K., Fassbender, K., Vandermeer, B., Durec, T., and Dryden, D.M. Granulocyte colony-stimulating factor for hepatitis C therapy-associated neutropenia: systematic review and economic evaluation. J Viral Hepat. 2011; 18: e381e393 [113]McHutchison, J.G., Dusheiko, G., Shiffman, M.L., Rodriguez-Torres, M., Sigal, S., Bourliere, M. et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007; 357: 22272236 [114]Homeida, S., Ebdon, C., Batty, P., Jackson, B., Kolade, S., Bateman, C. et al. New thrombopoietin receptor agonists for platelet disorders. Drugs Today (Barc). 2012; 48: 293301 [115]Schaefer, M., Capuron, L., Friebe, A., Diez-Quevedo, C., Robaeys, G., Neri, S. et al. Hepatitis C infection, antiviral treatment and mental health: a European expert consensus statement. J Hepatol. 2012; 57: 13791390 [116]Dalgard, O. Follow-up studies of treatment for hepatitis C virus infection among injection drug users. Clin Infect Dis. 2005; 40: S336S338 [117]Backmund, M., Meyer, K., and Edlin, B.R. Infrequent reinfection after successful treatment for hepatitis C virus infection in injection drug users. Clin Infect Dis. 2004; 39: 15401543 [118]Currie, S.L., Ryan, J.C., Tracy, D., Wright, T.L., George, S., McQuaid, R. et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus. Drug Alcohol Depend. 2008; 93: 148154 [119]Grebely, J., Pham, S.T., Matthews, G.V., Petoumenos, K., Bull, R.A., Yeung, B. et al. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012; 55: 10581069 [120]Grebely, J., Knight, E., Ngai, T., Genoway, K.A., Raffa, J.D., Storms, M. et al. Reinfection with hepatitis C virus following sustained virological response in injection drug users. J Gastroenterol Hepatol. 2010; 25: 12811284 [121]Bacon, B.R., Gordon, S.C., Lawitz, E., Marcellin, P., Vierling, J.M., Zeuzem, S. et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011; 364: 12071217 [122]Zeuzem, S., Andreone, P., Pol, S., Lawitz, E., Diago, M., Roberts, S. et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011; 364: 24172428 [123]Flamm, S.L., Lawitz, E., Jacobson, I., Bourliere, M., Hezode, C., Vierling, J.M. et al. Boceprevir with peginterferon alfa-2a-ribavirin is effective for previously treated chronic hepatitis C genotype 1 infection. Clin Gastroenterol Hepatol. 2013; 11: 81 ([e84; quiz e85]) [124]Singal, A.G., Volk, M.L., Jensen, D., Di Bisceglie, A.M., and Schoenfeld, P.S. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010; 8: 280288 ([288 e281]) [125]van der Meer, A.J., Veldt, B.J., Feld, J.J., Wedemeyer, H., Dufour, J.F., Lammert, F. et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012; 308: 25842593 [126]Schmid, M., Kreil, A., Jessner, W., Homoncik, M., Datz, C., Gangl, A. et al. Suppression of haematopoiesis during therapy of chronic hepatitis C with different interferon alpha mono and combination therapy regimens. Gut. 2005; 54: 10141020 [127]Garcia-Retortillo, M., Forns, X., Feliu, A., Moitinho, E., Costa, J., Navasa, M. et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002; 35: 680687 [128]Forns, X., Garcia-Retortillo, M., Serrano, T., Feliu, A., Suarez, F., de la Mata, M. et al. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003; 39: 389396 [129]Carrion, J.A., Martinez-Bauer, E., Crespo, G., Ramirez, S., Perez-del-Pulgar, S., Garcia-Valdecasas, J.C. et al. Antiviral therapy increases the risk of bacterial infections in HCV-infected cirrhotic patients awaiting liver transplantation: a retrospective study. J Hepatol. 2009; 50: 719728 [130]Everson, G.T., Trotter, J., Forman, L., Kugelmas, M., Halprin, A., Fey, B. et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005; 42: 255262 [131]Prieto, M., Berenguer, M., Rayon, J.M., Cordoba, J., Arguello, L., Carrasco, D. et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999; 29: 250256 [132]Forman, L.M., Lewis, J.D., Berlin, J.A., Feldman, H.I., and Lucey, M.R. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002; 122: 889896 [133]Berenguer, M., Palau, A., Aguilera, V., Rayon, J.M., Juan, F.S., and Prieto, M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008; 8: 679687 [134]Neumann, U.P., Berg, T., Bahra, M., Seehofer, D., Langrehr, J.M., Neuhaus, R. et al. Fibrosis progression after liver transplantation in patients with recurrent hepatitis C. J Hepatol. 2004; 41: 830836 [135]Blasco, A., Forns, X., Carrion, J.A., Garcia-Pagan, J.C., Gilabert, R., Rimola, A. et al. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. Hepatology. 2006; 43: 492499 [136]Samuel, D., Bizollon, T., Feray, C., Roche, B., Ahmed, S.N., Lemonnier, C. et al. Interferon-alpha 2b plus ribavirin in patients with chronic hepatitis C after liver transplantation: a randomized study. Gastroenterology. 2003; 124: 642650 [137]Carrion, J.A., Navasa, M., Garcia-Retortillo, M., Garcia-Pagan, J.C., Crespo, G., Bruguera, M. et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007; 132: 17461756 [138]Berenguer, M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008; 49: 274287 [139]Selzner, N., Guindi, M., Renner, E.L., and Berenguer, M. Immune-mediated complications of the graft in interferon-treated hepatitis C positive liver transplant recipients. J Hepatol. 2011; 55: 207217 [140]Garg, V., van Heeswijk, R., Lee, J.E., Alves, K., Nadkarni, P., and Luo, X. Effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology. 2011; 54: 2027 [141]Hulskotte, E., Gupta, S., Xuan, F., van Zutven, M., OMara, E., Feng, H.P. et al. Pharmacokinetic interaction between the hepatitis C virus protease inhibitor boceprevir and cyclosporine and tacrolimus in healthy volunteers. Hepatology. 2012; 56: 16221630 [142]Coilly, A., Roche, B., Botta-Fridlund, D., Leroy, V., Pageaux, P., Si-Ahmed, S. et al. Efficacy and safety of protease inhibitors for severe hepatitis C recurrence after liver transplantation: a first multicentric experience. J Hepatol. 2012; 56: S21 [143]Qurishi, N., Kreuzberg, C., Luchters, G., Effenberger, W., Kupfer, B., Sauerbruch, T. et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003; 362: 17081713 [144]Solas, C., Pambrun, E., Winnock, M., Salmon, D., Poizot-Martin, I., Dominguez, S. et al. Ribavirin and abacavir drug interaction in HIV-HCV coinfected patients: fact or fiction?. AIDS. 2012; 26: 21932199 [145]Alberti, A., Clumeck, N., Collins, S., Gerlich, W., Lundgren, J., Palu, G. et al. Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co-infected patients. J Hepatol. 2005; 42: 615624 [146]Opravil, M., Rodriguez-Torres, M., Rockstroh, J., Snoeck, E., Chung, R.T., Tietz, A. et al. The dose-response relationship of peginterferon alfa-2a and ribavirin in the treatment of patients coinfected with HIV-HCV. HIV Clin Trials. 2012; 13: 3345 [147]Rodriguez-Torres, M., Slim, J., Bhatti, L., Sterling, R., Sulkowski, M., Hassanein, T. et al. Peginterferon alfa-2a plus ribavirin for HIV-HCV genotype 1 coinfected patients: a randomized international trial. HIV Clin Trials. 2012; 13: 142152 [148]Dieterich D, Soriano V, Sherman K, Girard P-M, Rockstroh J, Adiwijaya B, et al. Telaprevir in combination with pegylated interferon-alfa-2a+RBV in HCV/HIV-co-infected patients: a 24-week treatment interim analysis. In: 19th conference on retroviruses and opportunistic infections, seattle, WA, March 58; 2012 [abstract 46]. [149]Sulkowski, M., Pol, S., Mallolas, J., Fainboim, H., Cooper, C., Slim, J. et al. Boceprevir vs. placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013; 13: 597605 [150]Kiser, J.J., Burton, J.R., Anderson, P.L., and Everson, G.T. Review and management of drug interactions with boceprevir and telaprevir. Hepatology. 2012; 55: 16201628 [151]European AIDS Treatment Network (NEAT) Acute Hepatitis C Infection Consensus Panel. Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS. 2011; 25: 399409 [152]Potthoff, A., Wedemeyer, H., Boecher, W.O., Berg, T., Zeuzem, S., Arnold, J. et al. The HEP-NET B/C co-infection trial: A prospective multicenter study to investigate the efficacy of pegylated interferon-alpha2b and ribavirin in patients with HBV/HCV co-infection. J Hepatol. 2008; 49: 688694 [153]Potthoff, A., Berg, T., and Wedemeyer, H. Late hepatitis B virus relapse in patients co-infected with hepatitis B virus and hepatitis C virus after antiviral treatment with pegylated interferon-a2b and ribavirin. Scand J Gastroenterol. 2009; 44: 14871490 [154]Fabrizi, F., Dulai, G., Dixit, V., Bunnapradist, S., and Martin, P. Meta-analysis: interferon for the treatment of chronic hepatitis C in dialysis patients. Aliment Pharmacol Ther. 2003; 18: 10711081 [155]Liu, C.H., Liang, C.C., Lin, J.W., Chen, S.I., Tsai, H.B., Chang, C.S. et al. Pegylated interferon alpha-2a vs. standard interferon alpha-2a for treatment-naive dialysis patients with chronic hepatitis C: a randomised study. Gut. 2008; 57: 525530 [156]Peck-Radosavljevic, M., Boletis, J., Besisik, F., Ferraz, M.L., Alric, L., Samuel, D. et al. Low-dose peginterferon alfa-2a is safe and produces a sustained virologic response in patients with chronic hepatitis C and end-stage renal disease. Clin Gastroenterol Hepatol. 2011; 9: 242248 [157]Liu, C.H., Liang, C.C., Liu, C.J., Tsai, H.B., Hung, P.H., Hsu, S.J. et al. Pegylated interferon alpha-2a plus low-dose ribavirin for the retreatment of dialysis chronic hepatitis C patients who relapsed from prior interferon monotherapy. Gut. 2009; 58: 314316 [158]Treitel, M., Marbury, T., Preston, R.A., Triantafyllou, I., Feely, W., OMara, E. et al. Single-dose pharmacokinetics of boceprevir in subjects with impaired hepatic or renal function. Clin Pharmacokinet. 2012; 51: 619628 [159]van Heeswijk, R., Vandevoorde, A., Boogaerts, G., De Paepe, E., van Solingen-Ristea, R., Garg, V. et al. The effect of severe renal impairment on the pharmacokinetics of the investigational HCV protease inhibitor telaprevir. J Hepatol. 2011; 54: S492 [160]Basu, P.P., Siriki, R., Shah, N.J., Farhat, S., Mittimani, K., Atluri, S. et al. Telaprevir with adjusted dose of ribavirin in nave CHC-G1: efficacy and treatment in CHC in hemodialysis population. Target C (RCT). J Hepatol. 2013; 58: S30S31 [161]Scott, D.R., Wong, J.K., Spicer, T.S., Dent, H., Mensah, F.K., McDonald, S. et al. Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation. 2010; 90: 11651171 [162]Gane, E. and Pilmore, H. Management of chronic viral hepatitis before and after renal transplantation. Transplantation. 2002; 74: 427437 [163]Van Wagner, L.B., Baker, T., Ahya, S.N., Norvell, J.P., Wang, E., and Levitsky, J. Outcomes of patients with hepatitis C undergoing simultaneous liver-kidney transplantation. J Hepatol. 2009; 51: 874880 [164]Martin, P. and Fabrizi, F. Hepatitis C virus and kidney disease. J Hepatol. 2008; 49: 613624 [165]Orens, J.B., Estenne, M., Arcasoy, S., Conte, J.V., Corris, P., Egan, J.J. et al. International guidelines for the selection of lung transplant candidates: 2006 update a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006; 25: 745755 [166]Grebely, J., Raffa, J.D., Lai, C., Kerr, T., Fischer, B., Krajden, M. et al. Impact of hepatitis C virus infection on all-cause and liver-related mortality in a large community-based cohort of inner city residents. J Viral Hepat. 2011; 18: 3241 [167]Darke, S., Kaye, S., and Duflou, J. Comparative cardiac pathology among deaths due to cocaine toxicity, opioid toxicity and non-drug-related causes. Addiction. 2006; 101: 17711777 [168]Shepard, C.W., Finelli, L., and Alter, M.J. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005; 5: 558567 [169]Hagan, H., Pouget, E.R., Des Jarlais, D.C., and Lelutiu-Weinberger, C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008; 168: 10991109 [170]Nelson, P.K., Mathers, B.M., Cowie, B., Hagan, H., Des Jarlais, D., Horyniak, D. et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011; 378: 571583 [171]Pybus, O.G., Cochrane, A., Holmes, E.C., and Simmonds, P. The hepatitis C virus epidemic among injecting drug users. Infect Genet Evol. 2005; 5: 131139 [172]van Asten, L., Verhaest, I., Lamzira, S., Hernandez-Aguado, I., Zangerle, R., Boufassa, F. et al. Spread of hepatitis C virus among European injection drug users infected with HIV: a phylogenetic analysis. J Infect Dis. 2004; 189: 292302 [173]de Bruijne, J., Schinkel, J., Prins, M., Koekkoek, S.M., Aronson, S.J., van Ballegooijen, M.W. et al. Emergence of hepatitis C virus genotype 4: phylogenetic analysis reveals three distinct epidemiological profiles. J Clin Microbiol. 2009; 47: 38323838 [174]van den Berg, C.H., Smit, C., Bakker, M., Geskus, R.B., Berkhout, B., Jurriaans, S. et al. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. Eur J Epidemiol. 2007; 22: 183193 [175]Mehta, S.H., Astemborski, J., Kirk, G.D., Strathdee, S.A., Nelson, K.E., Vlahov, D. et al. Changes in blood-borne infection risk among injection drug users. J Infect Dis. 2011; 203: 587594 [176]Patrick, D.M., Tyndall, M.W., Cornelisse, P.G., Li, K., Sherlock, C.H., Rekart, M.L. et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ. 2001; 165: 889895 [177]Maher, L., Li, J., Jalaludin, B., Chant, K.G., and Kaldor, J.M. High hepatitis C incidence in new injecting drug users: a policy failure?. Aust N Z J Public Health. 2007; 31: 3035 [178]Kim, C., Kerr, T., Li, K., Zhang, R., Tyndall, M.W., Montaner, J.S. et al. Unstable housing and hepatitis C incidence among injection drug users in a Canadian setting. BMC Public Health. 2009; 9: 270 [179]Roy, E., Alary, M., Morissette, C., Leclerc, P., Boudreau, J.F., Parent, R. et al. High hepatitis C virus prevalence and incidence among Canadian intravenous drug users. Int J STD AIDS. 2007; 18: 2327 [180]Bruneau, J., Daniel, M., Kestens, Y., Abrahamowicz, M., and Zang, G. Availability of body art facilities and body art piercing do not predict hepatitis C acquisition among injection drug users in Montreal, Canada: results from a cohort study. Int J Drug Policy. 2010; 21: 477484 [181]Aitken, C., Lewis, J., Hocking, J., Bowden, D., and Hellard, M. Does information about IDUs injecting networks predict exposure to the hepatitis C virus?. Hepat Monthly. 2009; 9: 1723 [182]Turner, K.M., Hutchinson, S., Vickerman, P., Hope, V., Craine, N., Palmateer, N. et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011; 106: 19781988 [183]Rehm, J., Frick, U., Hartwig, C., Gutzwiller, F., Gschwend, P., and Uchtenhagen, A. Mortality in heroin-assisted treatment in Switzerland 19942000. Drug Alcohol Depend. 2005; 79: 137143 [184]Kreek, M.J., Dodes, L., Kane, S., Knobler, J., and Martin, R. Long-term methadone maintenance therapy: effects on liver function. Ann Intern Med. 1972; 77: 598602 [185]Petry, N.M., Bickel, W.K., Piasecki, D., Marsch, L.A., and Badger, G.J. Elevated liver enzyme levels in opioid-dependent patients with hepatitis treated with buprenorphine. Am J Addict. 2000; 9: 265269 [186]Andreu, V., Mas, A., Bruguera, M., Salmeron, J.M., Moreno, V., Nogue, S. et al. Ecstasy: a common cause of severe acute hepatotoxicity. J Hepatol. 1998; 29: 394397 [187]Karch, S.B., Stephens, B.G., and Ho, C.H. Methamphetamine-related deaths in San Francisco: demographic, pathologic, and toxicologic profiles. J Forensic Sci. 1999; 44: 359368 [188]Hezode, C., Roudot-Thoraval, F., Nguyen, S., Grenard, P., Julien, B., Zafrani, E.S. et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005; 42: 6371 [189]Hezode, C., Zafrani, E.S., Roudot-Thoraval, F., Costentin, C., Hessami, A., Bouvier-Alias, M. et al. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology. 2008; 134: 432439 [190]Ostapowicz, G., Watson, K.J., Locarnini, S.A., and Desmond, P.V. Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology. 1998; 27: 17301735 [191]Martin, N.K., Vickerman, P., Miners, A., Foster, G.R., Hutchinson, S.J., Goldberg, D.J. et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012; 55: 4957 [192]Dore, G.J., Hellard, M., Matthews, G.V., Grebely, J., Haber, P.S., Petoumenos, K. et al. Effective treatment of injecting drug users with recently acquired hepatitis C virus infection. Gastroenterology. 2010; 138: e121e122 [193]Alvarez-Uria, G., Day, J.N., Nasir, A.J., Russell, S.K., and Vilar, F.J. Factors associated with treatment failure of patients with psychiatric diseases and injecting drug users in the treatment of genotype 2 or 3 hepatitis C chronic infection. Liver Int. 2009; 29: 10511055 [194]Conway, B., Grebely, J., Tossonian, H., Lefebvre, D., and de Vlaming, S. A systematic approach to the treatment of HIV and hepatitis C virus infection in the inner city: a Canadian perspective. Clin Infect Dis. 2005; 41: S73S78 [195]Grebely, J., Genoway, K.A., Raffa, J.D., Dhadwal, G., Rajan, T., Showler, G. et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008; 93: 141147 [196]Doab, A., Treloar, C., and Dore, G.J. Knowledge and attitudes about treatment for hepatitis C virus infection and barriers to treatment among current injection drug users in Australia. Clin Infect Dis. 2005; 40: S313S320 [197]Kramer, J.R., Kanwal, F., Richardson, P., Giordano, T.P., Petersen, L.A., and El-Serag, H.B. Importance of patient, provider, and facility predictors of hepatitis C virus treatment in veterans: a national study. Am J Gastroenterol. 2011; 106: 483491 [198]Gidding, H.F., Law, M.G., Amin, J., Macdonald, G.A., Sasadeusz, J.J., Jones, T.L. et al. Predictors of deferral of treatment for hepatitis C infection in Australian clinics. Med J Aust. 2011; 194: 398402 [199]Bini, E.J., Brau, N., Currie, S., Shen, H., Anand, B.S., Hu, K.Q. et al. Prospective multicenter study of eligibility for antiviral therapy among 4084 U.S. veterans with chronic hepatitis C virus infection. Am J Gastroenterol. 2005; 100: 17721779 [200]Kanwal, F., Hoang, T., Spiegel, B.M., Eisen, S., Dominitz, J.A., Gifford, A. et al. Predictors of treatment in patients with chronic hepatitis C infection role of patient vs. nonpatient factors. Hepatology. 2007; 46: 17411749 [201]Robaeys, G., Van Vlierberghe, H., Mathei, C., Van Ranst, M., Bruckers, L., and Buntinx, F. Similar compliance and effect of treatment in chronic hepatitis C resulting from intravenous drug use in comparison with other infection causes. Eur J Gastroenterol Hepatol. 2006; 18: 159166 [202]Hellard, M., Sacks-Davis, R., and Gold, J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009; 49: 561573 [203]Papadopoulos, V., Gogou, A., Mylopoulou, T., and Mimidis, K. Should active injecting drug users receive treatment for chronic hepatitis C?. Arq Gastroenterol. 2010; 47: 238241 [204]Manolakopoulos, S., Deutsch, M.J., Anagnostou, O., Karatapanis, S., Tiniakou, E., Papatheodoridis, G.V. et al. Substitution treatment or active intravenous drug use should not be contraindications for antiviral treatment in drug users with chronic hepatitis C. Liver Int. 2010; 30: 14541460 [205]Bruggmann, P., Falcato, L., Dober, S., Helbling, B., Keiser, O., Negro, F. et al. Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients. J Viral Hepat. 2008; 15: 747752 [206]Sasadeusz, J.J., Dore, G., Kronborg, I., Barton, D., Yoshihara, M., and Weltman, M. Clinical experience with the treatment of hepatitis C infection in patients on opioid pharmacotherapy. Addiction. 2011; 106: 977984 [207]Sylvestre, D.L., Litwin, A.H., Clements, B.J., and Gourevitch, M.N. The impact of barriers to hepatitis C virus treatment in recovering heroin users maintained on methadone. J Subst Abuse Treat. 2005; 29: 159165 [208]Mauss, S., Berger, F., Goelz, J., Jacob, B., and Schmutz, G. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004; 40: 120124 [209]van Heeswijk, R., Vandevoorde, A., Verboven, P., Boogaerts, G., De Paepe, E., van Solingen-Ristea, R. et al. The pharmacokinetic interaction between methadone and the investigational HCV protease inhibitor telaprevir. J Hepatol. 2011; 54: S491S492 [210]Luo, X., Trevejo, J., Van Heeswijk, R., and Garg, V. No significant effect of the HCV protease inhibitor telaprevir on pharmacokinetics and pharmacodynamics of buprenorphine in HCV-negative volunteers. Global Antivir J. 2011; 7: 116117 [211]Hulskotte, E., Feng, H., Bruce, R., Webster, L., Xuan, F., Lin, W. et al. Pharmacokinetic interaction between HCV protease inhibitor boceprevir and methadone or buprenorphine in subjects on stable maintenance therapy. J Gastroenterol Hepatol. 2012; 27: 169170 [212]Burger, D., Back, D., Buggisch, P., Buti, M., Craxi, A., Foster, G. et al. Clinical management of drug-drug interactions in HCV therapy: challenges and solutions. J Hepatol. 2013; 58: 792800 [213]Van Heeswijk R, Boogaerts G, De Paepe E, Van Solingen-Ristea R, Garg V, Beumont M. The pharmacokinetic interaction between escitalopram and the investigational HCV protease inhibitor telaprevir. In: Fifth international workshop on clinical pharmacology of hepatitis therapy, Boston, MA, June 2324; 2010 [abstract 12]. Дата добавления: 2016-03-26 | Просмотры: 685 | Нарушение авторских прав |