|
|
АкушерствоАнатомияАнестезиологияВакцинопрофилактикаВалеологияВетеринарияГигиенаЗаболеванияИммунологияКардиологияНеврологияНефрологияОнкологияОториноларингологияОфтальмологияПаразитологияПедиатрияПервая помощьПсихиатрияПульмонологияРеанимацияРевматологияСтоматологияТерапияТоксикологияТравматологияУрологияФармакологияФармацевтикаФизиотерапияФтизиатрияХирургияЭндокринологияЭпидемиология |
ACID-ALKALI BALANCE (AAB)
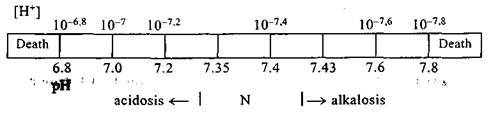
An acid is called a substance able to release hydrogen ion H+ (H+-donator). Alkali (base) is a substance able to bind hydrogen ion. In the organism the number of compounds having acidic and alkaline properties depends on the quantity and nature of consumed food, on the intensity of metabolic processes, on the way of removal of these substances from the organism. At the same time, a characteristic feature is inherent in the organism: definite concentration of ions of hydrogen are being maintained in a healthy human and this constancy is one of the most important components of biochemical equilibrium of liquid media of the organism — homeostasis. A change of these conditions leads to an alteration of physiologic systems of the organism, to disturbance of oxidative and other processes. A vital activity of the organism is associated, first of all, with continous exchange of substances. A high rate of metabolic processes is explained by availability of biologic catalyzers — enzymes the activity of which depends on the concentration of H+-ions. A shift of the concentration of hydrogen ions to any side from the norm leads to a significant decrease of enzymatic processes in the media of the organism and may lead to a fatal outcome. Thus, a constancy of the concentration of H+-ions in the organism is the absolute and esential condition of life. A ratio between bases and acids in the organism creates a normal pH value of blood 7.33-7.44. pH — it is a negative decimal logarithm of the concentration of hydrogen ions (for example, if the concentration is [H+= 10"7 mmol/1], then pH = lglO-7=7.0). An actual acidity is defined by the value of ratio OH"/H+. There is an excess There is an inverse relationship between the concentration of H+ and pH.
A shift of pH to the acidic side is called acidosis, to alkaline — alkalosis. A shift of blood to the side of acidosis may occur at the expense of: 1) excess of C02 in the organism; 2) excess of nonvolatile acids; 3) excessive elimination of bases from the organism. Blood may become alkaline at the expense of: 1) excessive elimination of C02 from the organism; 2) administration of the significant amount of alkalies into the organism. Buffer systems represent a mixture of weak acid and its salt formed by a strong base. There are four basic buffer systems being active in the organism: 1) bicarbonate — mixture NaHC03/H2C03; 2) hemoglobin Hb02/Hb. Oxygemoglobin Hb02 is a stronger acid than Hb. Giving back 02 in the tissues, hemoglobin loses a part of its acidic properties. So formed free Hb intensifies hemoglobin "affinity" to H+-ions and binds them. At the moment of C02 coming from the tissues into the blood Hb affinity to hydrogen ions drops. Thus, a reduced hemoglobin behaves itself in the organism as a base and shifts pH to the side of alkalosis, and oxyhemoglobin Hb02 behaves itself as an acid and shifts pH to the acidic side; 3) phosphate buffer system Na2HP04/NaH2P04; 4) protein buffer system. When acids or alkali are added to the buffer system, it changes its pH insignificantly or does not change it at all. Every buffer system has a definite power impeding a pH change, i.e. a capacity of buffer system. Under the capacity of the buffer system they understand such an amount of acid or alkali that is necessary to add to one liter of one-molar solution of the buffer system so as to change pH for 1 unit. The capacity of bicarbonate system is 0.3 1; hemoglobin one — 3.3 1; phosphate — 0.06 1. Thus, the most powerful is hemoglobin buffer system. So, when hemoglobin decreases blood becomes less stable to pH changes. For anesthesiologists a bicarbonate system is of significance since we may influence it: — to hold up or intensify a removal of H2C03, as well as through APV; — to introduce ordinary sodium bicarbonate into the organism. Mechanism of action of physiologic systems of AAB regulation lies in the elimination of a number of metabolites as a result of which AAB normalization occurs. The main organs ensuring a functioning of these systems are the lungs, kidneys, liver, GIT. Дата добавления: 2015-02-05 | Просмотры: 899 | Нарушение авторских прав |