 |
|
АкушерствоАнатомияАнестезиологияВакцинопрофилактикаВалеологияВетеринарияГигиенаЗаболеванияИммунологияКардиологияНеврологияНефрологияОнкологияОториноларингологияОфтальмологияПаразитологияПедиатрияПервая помощьПсихиатрияПульмонологияРеанимацияРевматологияСтоматологияТерапияТоксикологияТравматологияУрологияФармакологияФармацевтикаФизиотерапияФтизиатрияХирургияЭндокринологияЭпидемиология |
Preparations for acidosis correction
1. NaHC03 — sodium bicarbonate, molecular weight — 84 g. 1 ml of 8.4% soda solution contains 1 mmol of sodium bicarbonate, and 1 ml of 4% NaHCOj contains 0.5 mmol. Sodium bicarbnate is decomposed at the temperature of 60°C. Therefore, samples of soda powder are sterilized under ultraviolet radiation and prior to i/v administration are mixed ex tempore with apyretic water. Upon its administration sodium bicarbonate comes into interaction with organic To administer sodium bicarbonate for the patients with high pC02 is undesirable, because, in so doing, pC02 increases still more. Therefore, in respiratory acidosis NaHC03 is contraindicated! It is indicated in metabolic acidosis. 2. Sodium lactate, 11% solution. 1 ml of this solution contains 1 meq of alkali. It is well sterilized and stored. In i/v administration it comes into interaction with volatile acids forming lactic acid that undergoes a transformation in Krebs cycle with formation of 17 ATP molecules. Na lactate + H2C03 —> NaHC03 + l molecule of lactic acid This preparation is applied in respiratory acidosis when there is a possibility to burn up a lactic acid in Krebs cycle with energy generation. Therefore, sodium lactate serves as the preparation to cover energy demand. In tissue hypoxia when processes of metabolism in Krebs cycle are inhibited — sodium lactate is contraindicated!
3.66% solution, its 1 ml contains 0.3 meq of alkali. In the organism it comes into interaction with volatile and nonvolatile acids, therefore, it may be administered both in respiratory and metabolic acidosis.
However, it should be taken into account that in disturbance of excretory function of the kidneys a severe hyperkalemia is possible. It exerts its effect on respiratory center and in rapid administration leads to a respiratory arrest, therefore, it is infused slowly drop-by-drop and no more than 1.5 g/kg a day. A calculation of the deficit of buffer bases is carried out by Mellengard-Astrup formula: Deficit of buffer bases + patient's weight (kg) x 0.3 x [-BE] Example: patient's weight is 60 kg, BE = - 9 mmol/1. Deficit of buffer bases makes up 60 x 0.3 x (- 9) = - 162 meq of alkali. To eliminate the established deficit it is necessary to infuse: 162 ml 8.4% NaHC03, 1 ml of which contains 1 meq of alkali, or 162 ml 11% Na lactate, 1 ml of which contains 1 meq of alkali, or 486 ml 3.6% Tris-buffer, 1 ml of which contains 0.3 meq of alkali. Дата добавления: 2015-02-05 | Просмотры: 976 | Нарушение авторских прав |
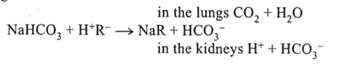 acids:
acids: 3. Trisamine (THAM)— tri-(oxymethyl)-aminomethane
3. Trisamine (THAM)— tri-(oxymethyl)-aminomethane