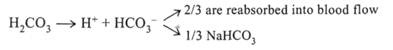|
|
АкушерствоАнатомияАнестезиологияВакцинопрофилактикаВалеологияВетеринарияГигиенаЗаболеванияИммунологияКардиологияНеврологияНефрологияОнкологияОториноларингологияОфтальмологияПаразитологияПедиатрияПервая помощьПсихиатрияПульмонологияРеанимацияРевматологияСтоматологияТерапияТоксикологияТравматологияУрологияФармакологияФармацевтикаФизиотерапияФтизиатрияХирургияЭндокринологияЭпидемиология |
Role of the lungs in AAB regulation
Free ions [H+] are not released with the lungs, however, strong acids, with the help of Na bicarbonate, are converted into a weak carbonic acid that dissociates down to C02 and C02 is a specific stimulator of respiratory center, dyspnea and hyperventilation arise and excessive amounts of C02 are eliminated with the lungs. In excessive accumulation of alkaline products an increased need arises in H2C03 that is spent for neutralization of these alkalies. When the supplies of H2C03 are reduced and [C02] concentration decreases the intensity of the respiratory center stimulation lowers: a hypoventilation arises that leads to a preservation of C02 in the organism and replenishment of H2C03 reserves in blood. When ventilation worsens and gas diffusion decreases, C02 is accumulated in blood and at the expense of this an excess of [H+] ions is accumulated in blood. pH decreases. In hyperventilation caused by stimulation of respiratory center (in CCC1, intracerebral bleeding) a primary dyspnea develops. The same condition may develop in improperly chosen parameters of APV. C02 is eliminated excessively and a respiratory alkalosis develops. In the norm PC02 = 40 mm Hg. When PC02 is less than the norm, a respiratory alkalosis arises, and if C02 is more than the norm — a respiratory acidosis results. The state associated with an excessive accumulation of nonvolatile acids or alkalies is called metabolic acidosis or alkalosis. Role of kidnevs in AAB maintenance lies in elimination of hydrogen ions from the blood with acidic properties and bicarbonate from the blood with alkaline properties.
Hydrogen ions are excreted with the urine, and Na+ enters the cells of tubules from the urine where it binds with HC03_ forming Na bicarbonate, the latter enters the blood alkalizing it. The kidneys can excrete H+ and reabsorb Na+ and bicarbonate at the expense of ammoniogenesis. Ammonia (NH3) is formed as a result of deamination of amino acids and easily diffuse through the membrane into tubular urine where it binds with hydrogen ions forming ammonium (NH^) that is not able to pass through a cellular membrane. In the urine ammonium binds with CI" ions and in the form of ammonium chloride is excreted with the urine. Na+, freed from CI", passes into the cells of renal tubules, binds with HC03" and then enters the blood. Reabsorption of bicarbonate also exists. In this case the cells of renal tubules absorb Na+ from the urine in exchange for H+. Hydrogen ion enters the urine where binding with bicarbonate it forms H2C03 that dissociates at once to C02 and H20. Almost all carbon dioxide diffuses into blood. Дата добавления: 2015-02-05 | Просмотры: 838 | Нарушение авторских прав |
 H20:
H20: A resultant acidosis is called as gaseous or respiratory since it is associated with accumulation of volatile acids.
A resultant acidosis is called as gaseous or respiratory since it is associated with accumulation of volatile acids.