|
|
АкушерствоАнатомияАнестезиологияВакцинопрофилактикаВалеологияВетеринарияГигиенаЗаболеванияИммунологияКардиологияНеврологияНефрологияОнкологияОториноларингологияОфтальмологияПаразитологияПедиатрияПервая помощьПсихиатрияПульмонологияРеанимацияРевматологияСтоматологияТерапияТоксикологияТравматологияУрологияФармакологияФармацевтикаФизиотерапияФтизиатрияХирургияЭндокринологияЭпидемиология |
Metabolic alkalosis
It develops in case of loss of nonvolatile acids or loss of K+ that leads to the excess of H+ excretion by the kidneys. pH and concentration of HC03", PC02, SB and BE are increased, K+ and Cl" are decreased. Metabolic alkalosis is compensated by respiratory acidosis, and this compensation is weakly expressed. Here, K+ leaves the cells for plasma, and H+ moves into the cell with development of intracellular acidosis in plasma alkalosis. The main causes of metabolic alkalosis: 1. Deficit of K+ as a result of its losses or restriction of entering. 2. Losses of H+ and Cl" in recurrent vomiting and gastric drainage. 3. Introduction of the excess of bases or sodium citrate in massive blood transfusions. 4. Oliguria with retention of sodium and bicarbonate after traumas, operations. 5. Prolonged and uncontrolled administration of diuretics leading to washout of K+ and Cl~. 6. Prolonged application of steroid hormones. Described shifts of AAB and content of ions in plasma are generalized in Table 25. Table 25 Direction of shifts of AAB of blood and ions of plasma
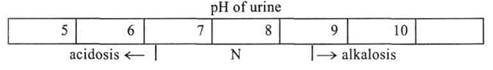
One of the auxiliary methods of AAB assessment is a determination of reaction of urine. In order to do this a litmus paper is usually used that changes its colour in pH 7.0. However, in the norm a reaction of urine varies within 6.5-8.5 (Fig. 44). Therefore, it is more expedient to determine the reaction of urine by means of pH-meter.
Дата добавления: 2015-02-05 | Просмотры: 813 | Нарушение авторских прав |